
Weekly Biotech Review Part I
Covering:
- Titan Pharmaceuticals
- Oramed Pharmaceuticals
This week begins a marathon of critical FDA decisions for 6 different companies. We will cover four of them here. Let’s begin with Titan Pharmaceuticals, Inc. (NASDAQ:TTNP)
Titan Pharmaceuticals
On May 27, the FDA will issue its final decision on Probuphine for the maintenance treatment of opioid addiction. Drug addicts, whether of the legal kind from painkillers like Vicodin prescribed by licensed doctors, or the illegal kind like heroin or other non-government-sanctioned opioids, generally need help with their chemical dependencies. Probuphine is not a new drug, but a new way of delivering it. Probuphine is a subcutaneous insert loaded with buprenorphine, the most widely prescribed opioid substitute for morphine, heroin, and other opiate addictions selling $2 billion a year in the United States. Like methadone, buprenorphine delivers relief from withdrawal without producing the associated high. Think of it as a nicotine patch implant for opioid addicts instead of cigarette addicts.
The Science
Opioid addiction is characterized as a medical condition that is already at pandemic proportions. So much for the war on drugs, it isn’t working. Addicts typically get either methadone or buprenorphine, but the latter is preferred because there are fewer issues of overdose and respiratory suppression. Buprenorphine can only suppress breathing so far, no matter the dose. Buprenophine overtook methadone as of 2006, partially for this reason. Opioid overdose causes death by suppressing breathing, as it acts on receptors in the medulla that control breathing and heart rate.
The currently most popular form of buprenorphine is sublingual. Patients take the drug under their tongue and from there it is absorbed into the blood stream. The disadvantages of this method mainly stem from the fact that dosage is volatile and moves up and down within loose borders. If you’re an addict trying to live a normal life, this makes it hard to operate normally, as sometimes there is not enough drug in your system to combat withdrawal symptoms, and sometimes there is too much, making you drowsy and unproductive.
Worse, the US government for some reason puts a cap on the number of people that can be prescribed buprenorphine, probably because it wants to believe it still has some sort of control over the war on drugs, which it does not. On the plus side for Probuphine, the Department of Health and Human Services has gracefully decided to expand the quota of patients that can be prescribed the opioid substitute, which will increase the total dollar amount of buprenorphine prescribed from $2 billion a year to something higher, though we don’t know the figures yet.
Probuphine is based on a technology that Titan calls ProNeura. It’s basically a small foam rubber stick that is made of a mixture of ethylene-vinyl acetate (EVA) and whatever drug they want to deliver, in this case buprenorphine. The insert is surgically implanted in the upper arm and is then removed after 3 to 6 months depending on the dosage program. Probuphine, unlike sublingual delivery, gives the patient a steady amount of drug that does not change for however long the implant has the active ingredient in it. EVA does not dissolve and is a very stable substance and hypoallergenic. The drug, however, does dissolve slowly, at a constant rate, no peaks and troughs.
The Data
Probuphine has been on the chopping block once before. Early in 2013, it was under review for final approval, after an FDA panel had actually voted in favor of its approval. Unfortunately and to the shock of stakeholders, the FDA spurned the advice of its review panel and decided to reject the drug for various reasons we will go into shortly. This collapsed Titan’s market cap and left investors reeling, and left the relevance of FDA review panels an open question since they don’t have to be listened to anyway.
The vote was not unanimous however at 12 to 5, and the FDA must have been concerned by some opinions on the panel, including that of one Dr. Tracy Rupp, who said there was not enough evidence to instruct doctors on how to insert the implant. She also noted that only 8% of patients tested negative for other opiods after four months of treatment with Probuphine, meaning the rest were cheating. She also noted that it took four weeks for drug levels to reach optimal concentrations, during which period patients were probably undergoing withdrawal and risked relapse.
There were other issues as well, including the Risk Evaluation and Mitigation Strategy or REMS, a new power the FDA has as of a 2007 law that is too bureaucratic to break down here. In any case, after the rejection which frankly surprised Titan and its partner Braeburn Pharmaceuticals, there were other meetings and supposedly all parties are on the same page now, but who knows.
In their words, following a meeting with the FDA in November 2013, there was general agreement on the next steps which included the final Phase 3 study and validation of the training program through human factor testing. Everything was completed, but that wasn’t the end of the story.
Probuphine originally had a review date of February 27 this year, but was extended another three months to May 27 due to more changes in the REMS section that constituted a major amendment. When we get to a mixture of bureaucracy and science over regulations like these, it becomes a guessing game. Was the issue, whatever it was, solved this time for good or not? Maybe, but that’s what everyone thought last time when the FDA panel voted to approve but the agency in the end rejected approval anyway.
In any case, the latest Phase 3 trial showed that participants who were clinically stable on sublingual buprenorphine maintained stability when transferred to Probuphine over six months and that they were more likely not to cheat by taking extra narcotics compared to those who kept taking the sublingual version. Since the data were not really the issue last time and the decision was more weighted towards implantation, REMs, and the method and timeline of transfer from sublingual to subcutaneous implant, the success of the data does not really matter here in terms of investment. It works. We know that. But will the FDA approve it this time? With a bureaucracy as mercurial as the FDA, who knows. We would say probably yes, because if it is rejected this time, most companies will shy away from developing any drug implants for fear that all the capital involved will be flushed down the toilet.
But even so, there is still a potential marketing problem with Probuphine.
The Market
As we mentioned earlier, the market for buprenorphine is currently at $2 billion in the US and set to rise due to loosening restriction over patient quotas. Titan will not be getting 100% of the proceeds though, as they are partnered with private firm Braeburn Pharmaceuticals. They will get $15 million as a milestone payment upon approval and royalties in the mid-teens to low twenties.
Further, the REMS requirement that was the cause of so many problems in 2013 can affect the marketability of a drug. If doctors have to undergo training to implant the device and take a course about who to prescribe, it will discourage doctors from prescribing it.
The main problem though is that we have no idea how many patients will be willing to get themselves implanted with a piece of foam rubber for 3 to 6 months. Taking the infamous example of Mannkind Corporation’s (NASDAQ:MNKD) inhalable insulin Afrezza, which seemed like a good idea but was rejected by the market so far, addicts may prefer taking something sublingually rather than going to a doctor for a surgical procedure, advantages of Probuphine notwithstanding.
So even if Probuphine is approved, its performance in the market is far from assured. Braeburn itself has no approved products on the market, so as a firm it does not have substantial marketing experience. In a sentence, we have no idea how much Probuphine will sell, due to the nature of it as an implant and REMS requirements and the fact that its marketer is not very experienced.
Market Reaction
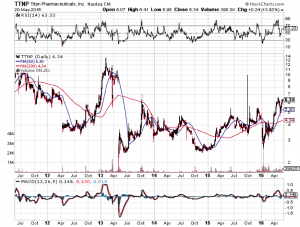
In 2013 Titan’s stock went to $12.50 when an FDA panel voted to approve the drug, but it was still rejected. This time the positive panel vote did not inspire any sustained reaction, fool me twice as they say. There has been a steady rise as optimism increases though. If the FDA rejects it again, Titan could be doomed because investors will start asking whether the FDA will ever approve any long term implants, which is Titan’s entire business.
If Probuphine is rejected again, it will probably be the end of Titan. If it is approved, look for the stock to go near previous highs, but not for long as marketing realities will quickly set it. That said, the trading strategy for this one is to stay away if you are risk averse. If you have extra pocket cash hanging around your account though, a very small position could get you some significant short term gains, but don’t hold it long after approval at all, because it will probably fall back down within months or maybe even weeks, certainly after the first sales report is released post approval.
Since the existence of Titan as a going concern depends on Probuphine’s approval, the financial clock is immaterial. It’s do or die now for Titan.
BY: RAFI FARBER AND SAMUEL RAE




