In Donald Trump’s latest press conference, the President-elect took a swipe at the healthcare and pharmaceutical space, suggesting that drug companies are getting away with murder, and hinting at some sort of legislation to limit said companies’ ability to charge the (admittedly exorbitant) prices they currently list. True to form, the biotech sector has taken a hit on the conference, and a number of the big names are down multiple percentage points on expectations that any legislation would limit profitability. Now, we aren’t saying that said legislation wouldn’t impact these companies, but we’ve seen this all before. Since mid-2015, political rhetoric has essentially controlled sentiment in the biotech space, and any actual legislation is yet to materialize. Trump has plenty to deal with before he gets to drug pricing, and we expect it will be a while before anything is set in stone. As such, there looks to be something of an opportunity to pick up some exposures at a discount on the President-elect’s latest inference. Bristol-Myers Squibb Co (NYSE:BMY), AstraZeneca (NYSE:AZN) and Roche Holding Ltd. (OTCMKTS:RHHBY) are all down, and an entry on the pullback could be a quick turnaround position.
With that said, here’s another that isn’t as politically swayed, and that could move this quarter. The company is ZIOPHARM Oncology Inc. (NASDAQ:ZIOP), and it just gave markets a pretty detailed update as to its operations and forward plans at the JP Morgan Healthcare Conference.
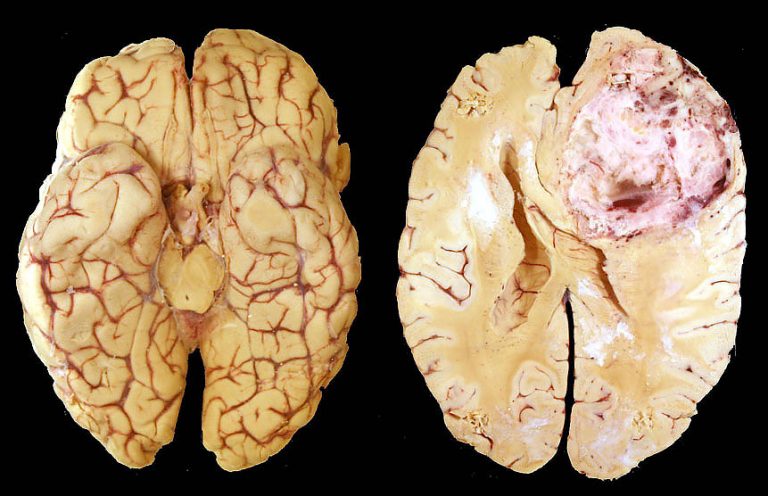
What we are interested in, is a drug called Ad-RTS-hIL-12. It’s not a particularly catchy name, but we’re guessing that will change near term. Anyway, the drug just completed a phase 2 study in a glioblastoma multiforme (GBM) indication, and the data looked excellent. GBM is an extremely tough condition to treat. It’s the most aggressive form of brain cancer, and there’s basically no cure. Patients diagnosed with the condition will undergo chemotherapy and radiotherapy (and if lucky, some degree of surgical resection) but life expectancy is very short, and generally subject to the debilitating side effects of a chemotherapy regimen. The mechanism of action for this drug is pretty complicated, but essentially, a physician injects the Ad-RTS-hIL-12 into the tumor, and it acts as a sort of switch to turn on the production of what’s called IL-12. This is an interleukin that is naturally produced by dendritic cells, and plays a key role in immune response. At the same time as the injection, the patient takes an activator pill (in this instance, a drug called Veledimex) and the combination of the IL-12 switch and the activator sparks an immune response targeting the tumor in question. In a GBM indication, of course, the tumor is the brain tumor.
Based on interim results from the phase 2 study, the company noted that the combination treatments is well tolerated, and suggests a survival benefit over historical controls at six, nine and 12 months, with a median overall survival of 9.6 months across a 25 person patient population. Additionally, the company noted that there was a strong correlation between the dosing and the IL-12 production (suggesting that a high dose results in a high immune response) and that the gene switch works as a way to turn on and off the production of IL-12. Across 15 patients, and as of January 6, 2017, the drug combination resulted in a median overall survival of 12.7 months. This blows pretty much every other drug that has gone through clinical studies out of the water, with the closest being Temozolomide, which registered median overall survival of around nine months in 68 patients.
So there is plenty of data suggesting that this drug is both effective, and tolerable, but what is the catalyst, and why are we highlighting it now? Well, the company is set to sit down with the FDA for an end of phase 2 face-to-face meeting early this quarter. For us, this suggests the meeting will take place at some point over the next few weeks. The outcome of the meeting, with any luck, will be a clear pathway to commercial acceptance, by way of a solid pivotal trial design. The pivotal is set to kick off at some point during 2017 (we are expecting late second or early third-quarter), meaning there is a flurry of essential catalysts set to hit press between now and the end of the year for Ziopharm.




