This Week in FDA vs Biotech Part I, Will Teva’s Vantrela Get Abuse-Deterrent Label?
by: Rafi Farber and Samuel rae

Based on our research, there is a bit of confusion here as to the FDA committee’s opinion on Vantrela’s abuse deterrence. According to mainstream news sources primarily emanating from Reuters and being syndicated from there, the committee believes Vantrela does not deter abuse when taken orally, but only when crushed and snorted or dissolved and injected. When manipulated and swallowed, apparently the abuse deterrence is less effective.
The problem is, the briefing document available on the FDA website to does not directly state FDA’s opinion on the abuse deterrence of Vantrela when taken orally. There are some indications in the data that manipulation followed by oral administration is not as effective at deterring abuse as dissolving the drug in a tincture, isolating and injecting, but the data does show some level of effectiveness with manipulated oral administration. So whether the statement that Vantrela ER “does not have abuse-resistant properties when taken orally, according to a preliminary review by the U.S. Food and Drug Administration” in the words of Reuters is speculation, word of mouth or something else is unclear.
In all likelihood though, it is based on something if not explicitly in a written document, so it’s probably a no-go on the abuse-deterrent label.
The Science
Teva calls the mechanism behind its ADF proprietary, so there isn’t all that much to reveal about it. It was bought by Teva when it acquired Cephalon in 2011, which owned a biotech called CIMA which developed the tech and called it OraGuard™. Teva now just calls it the technology itself after the company that developed it, CIMA, ditching the OraGuard name.
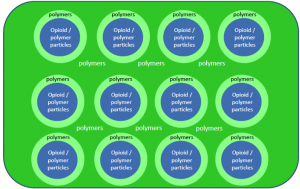
The basic concept is this, taken from an old Teva research and development presentation:
It is a polymer/opiod mixed core surrounded by polymers, surrounded by polymers again, an enigma wrapped in an enigma wrapped in a vest as Lisa Simpson once said of Nelson Muntz. The triple-layer of polymers prevents the active ingredient from being isolated through crushing and snorting or dissolving in alcohol. Extrapolating on how this might work even though it is proprietary, since “like dissolves like” meaning polar molecules dissolve polar molecules and nonpolar dissolves nonpolar, and alcohol is a polar molecule, the polymers that surround the active ingredient should be nonpolar so they won’t dissolve in alcohol.
If that is true, then some of the active ingredient should be able to be dissolved in a nonpolar solvent like butane, a common cooking fuel and an easily obtainable material that cannabis manufacturers often use to isolate tetrahydrocannabinol from cannabis to make concentrates. There are three kinds of barriers to this ADF technology, so it might be a mix of different kinds of molecules that can resist both polar and nonpolar solvents, but Teva is tight-lipped on that. If it is a mix, then the only way to abuse it for dose-dumping would be to dissolve in nonpolar and then polar solvents alternately or a solvent mix until all the polymer layers are dissolved. Since there are physical barriers as well to dissolution, it would require very high temperatures, which would denature the active ingredient upon extraction. As we’ll see, Vantrela was able to maintain its abuse-deterrent properties even with advanced solvents because even though the drug was extracted via advanced methods, the purity of it was very low once extracted, probably due to the high temperatures necessary to do it.
The Data
For those who want to see the whole dataset, it is available at the FDA briefing link above, section 1.3 on page 17. Picturing how abuse-deterrence following simulated abuse is tested is actually a bit morbidly comical. Basically, after it is tested with tools and solvents, they have experienced opioid users crush it and snort it etc. and then report their “drug liking” levels at various stages post use.
A quick summary, one Phase III study was double-blind and placebo-controlled for withdrawal on a dose of 30 to 90mg every 12 hours. This followed an open label phase where everyone took the active drug, so that the double-blind phase was able to measure withdrawal compared to placebo, measuring worst pain intensity measurements for both arms. Patients who continued receiving Vantrela maintained their improved scores on pain, while patients on placebo had their pain come back. The numbers were .07 baseline for the Vantrela arm and 0.71 for placebo, meaning placebo pain was 10 times worse than Vantrela.
Since the active ingredient is the same, just surrounded with polymers, there were no significant safety differences between Vantrela and other approved oral opiates. Safety should not be an issue here any more than other opioids.
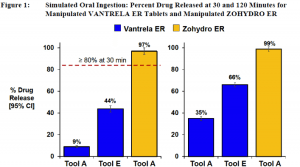
As for the abuse deterrence results, simulating digestion showed that Vantrela was much more resistant to abuse than Zohydro ER from Zogenix, Inc. (NASDAQ:ZGNX), a hydrocodone formulation that was approved in 2015 but without an abuse-deterrent label despite the FDA advisory committee’s vote against approval entirely. Both Zogenix and Vantrela are free of acetaminophen to prevent liver toxicity, so both are direct competitors.
Simulated digestion yielded this graph. Abuse deterrence is defined as less than 80% drug release in 30 minutes.
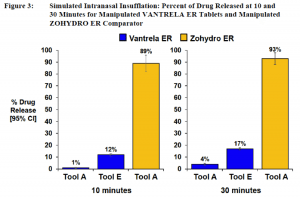
Simulated snorting, what they call insufflation to sound more scientific and less like a kingpin’s hangout, yielded these results:
The tests continued with all kinds of unidentified solvents and temperatures to simulate purification for injection. Vantrela beat Zohydro in every category. What makes Vantrela abuse-deterrent in even advanced solvents is that its purity declined as more and more of the drug was extracted. This might have to do with the temperatures required to break through the polymer layers that denatures or destroys the active ingredient once it is isolated, like a self-destruct mechanism.
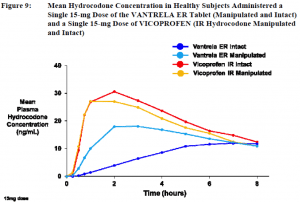
So after all this testing, what is the Reuters source talking about when it says that a preliminary review holds that Vantrela is not abuse-deterrent when taken orally? Perhaps it is this:
The graph above shows manipulated Vantrela versus an immediate release opiate. We can see from the graph that manipulation does do something to release more active ingredient faster, but not fast enough to qualify as dose dumping.
Market, Reaction, and Trading
As we detailed in our coverage of Collegium Pharmaceutical Inc. (NASDAQ:COLL) and its own abuse-deterrent opioid Xtampza ER, Oxycodone sells about $2 billion a year. Xtampza has target revenues of about $700 million. Assuming Vantrela has similar targets, it would add about 3.6% to Teva’s top line revenues. That’s a nice chunk, but not terribly important for the giant.
Due to the Reuters report that says FDA has a negative opinion of Vantrela’s abuse-deterrence when taken orally, the market is now expecting a negative review by the panel at least in regards to an abuse-deterrent label. However, there is nothing that we see in the briefing document, besides that one graph just above, that indicates that this is actually FDA’s opinion, unless Reuters is referring to a different release on the FDA website.
Even if it is true though that the advisory committee is going to vote no on abuse-deterrent labeling, there is still last year’s example of Zohydro ER which was approved over the advice of the advisory panel, though without abuse-deterrent labeling. This could hint at FDA bias towards leniency when it comes to abuse-deterrent opioids, given that opiate abuse is at epidemic proportions in the United States. Approval is almost a given. And even if the committee votes no on the label, the FDA may in the end grant the abuse-deterrent label anyway.
The committee’s verdict should not negatively affect the stock if the label is shot down, especially with current expectations, so investors are protected from downside if the label vote is negative. Upside is much higher if they do get a good verdict on the label unexpectedly, and given the data the chances are not all that bad. All things considered, there is little reason not to buy Teva now, or hold it if you already own it. In any case it should not be sold just because of the Reuters report.