While most westerners don’t have to worry about hepatitis B virus (HBV) due to currently available and effective vaccines, HBV is still an incurable disease, with a staggering 240 million people currently chronically infected worldwide. Of those infected, over 686,000 people die every year from HBV complications. Aside from vaccines, which are ineffective once infection has taken hold, Gilead Sciences (NASDAQ:GILD) has Viread, one of the best selling treatments for HBV that sold over $1.1B in 2015. But Viread is still not a cure.
Gilead’s stock has also been in decline of late, and the company is looking for a shot in the arm.
Plenty of analysts have discussed possible comeback routes for Gilead, but there is one wild card that is getting almost no attention. Spring Bank Pharmaceuticals (NASDAQ:SBPH) is a newly public biotech that saw a tepid IPO this past year, and is pursuing something quite bold – not just another treatment for chronic HBV, but a functional cure. That means consistently undetectable levels of virus in the blood. Gilead’s and Spring Bank’s technologies are complementary and being tested together in clinical trials, and the combination may prove to be the end of HBV as a chronic illness.
A successful combination treatment between Spring Bank’s SB9200 and Gilead’s Viread in Phase II trials may take HBV treatment over the top. The two companies announced their collaboration back in June 2015. The trial began this June and will announce top line results for the first cohort treated with only SB 9200 in early 2017, but the more important results will be from those sequentially treated with SB 9200 and then Viread. Those results are slated for the second half of 2017 and would be the first evidence of a functional HBV cure in humans if successful. Any strong efficacy results from the sequential cohorts would greatly increase Spring Bank’s chances of a buyout, perhaps even by Gilead, even before FDA approval of SB 9200.
Gilead and HBV
The HBV is not Gilead’s bread and butter, Gilead does have a strong pipeline of drug development for hepatitis B, with three simultaneous clinical trials at the end of 2015. Late last year, Gilead suffered a setback in a HBV clinical trial when one of those three trails, a Phase II therapeutic vaccine being developed in conjunction with GlobeImmune, failed. Gilead’s second HBV trial is only an improved version of their market leading Viread, so it doesn’t really ‘count’ as an innovation from a marketing and strategic standpoint.
With a weakening HBV pipeline, Gilead may be looking to retain its dominance in the HBV marketplace by collaborating with Spring Bank. Interestingly, Chief Medical Officer of Spring Bank, Dr. Nezam Afdhal, also currently serves on the Scientific Advisory Board of Gilead Sciences and receives research support and consulting fees from Gilead as well, showing the possibility of a closer union between the two firms. Dr. Afdhal’s public disclosures as an officer of Spring Bank shows him owning stock in both companies. This shouldn’t be surprising since he serves both, but does show his belief in this combination.
A Manual Interferon Burglar Alarm
Unlike Gilead’s Viread, which is a direct-acting antiviral medication, Spring Bank’s SB 9200 is an immunomodulatory therapy. Its job is to enhance the body’s own inherent ability to fight the virus by activating immune responses that HBV normally turns off. The idea is synergy, with SB 9200 first beefing up security and then Viread coming in and dealing HBV the final blow.
It should be kept in mind that there really is no “cure” for any virus, as one would cure say a bacterial infection. Viruses stay dormant in one way or another in the body, many times within DNA, even common colds. So what the two companies are looking for here is what’s known as a functional cure, meaning no virus detectable in the blood for three months or more. After a functional cure, no more antiviral medication should be needed.
The functional way in which SB 9200 enhances the immune system is as follows. HBV has an ability to suppress the body’s normal interferon signaling system. Interferon is normally activated by a virus-infected cell, which in turn lets all surrounding cells know it is infected. The body kicks its immune response into high gear, attempting to fight the virus and kill infected cells. But when HBV infects a cell, it tends to deactivate the alarm system. SB 9200 looks like a mock-DNA molecule, which tricks the body into thinking that interferons have indeed been released, effectively sounding the burglar alarm manually.
How Much HBV Does a Woodchuck Chuck?
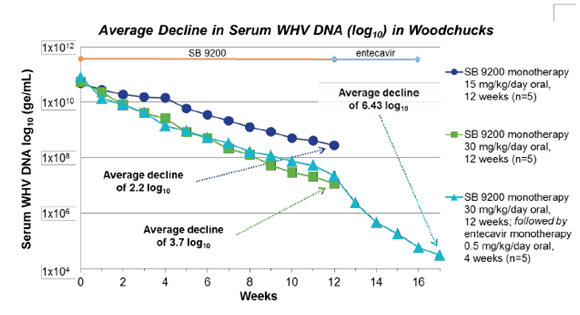
But what hard evidence do the companies have that a SB 9200 Viread sequential combination would work? First, they have woodchucks. Woodchucks can catch a very similar form of hepatitis to humans called, appropriately, the woodchuck hepatitis virus (WHV). It is almost identical to human hepatitis. The results on WHV-infected woodchucks were encouraging. All animals given SB 9200 responded to the drug at some level in a dose-dependent manner, and the addition of antiviral entecavir after SB 9200 increased potency. Lastly, no harmful side effects were observed. These results warranted a human-based clinical trial, giving rise to a Phase I trial.
In the Phase I trial on HCV patients, Spring Bank planned to show that interferon signaling was restored with SB 9200 as a monotherapy. Although not all patients responded, those that did showed activated interferon gene signaling as expected, with once again dose-dependent viral load reductions. While this doesn’t yet prove the efficacy of SB 9200, it is evidence of a unique mechanism of action that can act along with other antivirals. No adverse events were observed here either.
Phase II trial and some new investors
After the results of Phase I, some more attention was given to Spring Bank. Most notable was of course Gilead, which saw the potential for complementarity post trial. Additionally, last month, a shot in the arm was given to Spring Bank in the form of a $15 million dollar investment in the company by a consortium of companies led by MPM Capital.
On top of that, on December 19th, Arbutus BioPharma Corp (NASDAQ:ABUS), a smaller $150M company focused on HBV, also signed a partnership deal with Spring Bank also involving SB 9200. This will be in combination with its own experimental AB 423, which blocks capsid assembly, one of the essential steps in viral replication. AB 423 has a unique mechanism of action to HBV drugs on the market today, and though the collaboration is experimental, it does show that companies of all sizes from Big Pharma to trial stage are keeping an eye on Spring Bank’s pipeline. Meaning, if it works with one partner, these partnerships are likely to ramp up.
What should one expect from the Phase II trials which began six months ago? Different dosages of SB 9200 (25 – 200 mg) will be given to patients with HBV followed by Gilead’s Viread. As Viread’s efficacy is statistically well known, this Phase II study will aim to show if the levels of HBV decrease faster using combination therapy. Full results of the sequential study study are due by the end of 2017. The results of this study can very well change the face of Spring Bank, as Gilead may become interested in testing SB 9200 with its HCV drugs as well, which are its bread and butter.