On September 14, 2016, the FDA’s Oncologic Drugs Advisory Committee met to evaluate and discuss a drug called apaziquone. Apaziquone is a development stage bladder cancer treatment, which small cap biotech Spectrum Pharmaceuticals, Inc. (NASDAQ:SPPI) is investigating as a therapy designed to reduce the risk of recurrence of bladder cancer in patients that have juts undergone surgery to remove small, low grade, bladder cancer tumors. The advisory panel voted against approval, and the FDA will report its final ruling by way of a December 11, 2016 PDUFA.
Ahead of the PDUFA, here’s our take on apaziquone, and an unwinding of the briefing document put together by the agency.
First up, let’s take a look at the indication – bladder cancer.
The drug is targeting hat’s called non-muscle invasive bladder cancer (NMIBC), which as its name suggests, is a type of bladder cancer that is limited to, and bound by, bladder tissue, and which has not spread to surrounding muscle tissue. Specifically, in this instance, apaziquone is designed to treat a low grade of the disease, as defined by the International Society of Urological Pathology 2004 WHO grading system:
Low risk disease includes stage Ta, low grade lesions that are < 3 cm. Patients with multiple or recurrent lesions or who have carcinoma in situ are not considered to have low-risk disease.
I the US, an estimated 76,960 adults (58,950 men and 18,010 women) will be diagnosed with bladder cancer each year, and 6,390 deaths (11,820 men and 4,570 women) from the disease occur every year. It is the fourth most common cancer among men, and for reasons as yet undetermined (at least, that is, with any degree of certainty) it’s far more prevalent among men than women.
The current standard of care therapy for this degree of bladder cancer is resection – a surgeon goes in and cuts out as much of the cancerous matter as possible, with the goal obviously being to remove all the tumor cells. There’s a real issue with recurrence, however, and it primarily revolves around the cells that come loose while the physician is cutting away the primary tumor or tumors. These cells lodge back in to the bladder tissue, and form fresh tumors.
In an effort to avoid this recurrence, some physician will use off label chemotherapy drugs as a sort of post-surgery wash out, flushing the bladder with the drugs in question in an attempt to kill any erroneous cancer cells. We’re going to discuss this in a little more detail shortly, but for reference, the drugs most used are epirubicin and mitomycin – to well established and widely used chemo agents. It’s important to note here that the use of these two drugs in this instance, what’s called intravesical chemotherapy, is not approved by the FDA. Physicians administer the intravesical chemotherapy to minimize the chances of recurrence, but there have been no trials (to date) that prove the method is safe and effective. It’s assumed safe, based on the established nature of the drugs in question, and the efficacy assumption is based on meta analysis.
So with that out of the way, let’s move on to apaziquone.
Apaziquone (which Spectrum is referring to by trade name EOquin) is what’s called an indolequinone. It is a bioreductive prodrug, which means it remains inactive until it reaches its target location, and a chemical analog of the above mentioned, and well established. chemotherapeutic agent mitomycin. The cells that make up the inner surface of the urinary bladder are a type of cells referred to as hypoxic, which just means they are deprived of oxygen. When the drug comes in to contact with a hypoxic cellular environment, it is converted to active metabolites by intracellular reductases. The active metabolites alkylate DNA and initiate a process called apoptotic cell death. For those not familiar with apoptosis, it’s the process through which cells die. It generally involves a break down of the cell membrane, which allows the intracellular material to leak out of the cell, followed by a clean up process involving white blood cells – specifically, macrophages. In healthy cells, apoptosis takes place at a pretty regular rate, and it’s the standard process through which cells remove themselves from our system on expiry. Cancerous cells don’t initiate apoptosis naturally, and this leads to the excessive proliferation associated with the disease.
Jumping back to the currently used treatments, the above mentioned meta analyses that hint at efficacy for epirubicin and mitomycin when used as intravesical chemotherapy agents immediately post-surgery suggest a reduction in recurrence of somewhere between 5-15%. The standard accepted number is 12%. To put this another way, if a surgeon flushes a patient with either epirubicin or mitomycin once he or she has removed the tumors from the patient’s bladder, evidence suggests that patient has a 12% smaller chance of fresh bladder cancer tumors forming than they would otherwise have without the intravesical chemotherapy.
There are a few things to note here.
The first, that 12% is not that high. Especially in a low grade severity cancer, a reduction of 12% is not enough to warrant SOC status for these off labels. The second is that the 12% figure is an optimistic estimate. The third, that many physicians don’t use intravesical chemotherapy post resection, for the simple reason that they don’t class the 12% figure as providing any meaningful clinical benefit. This latter point, clinical benefit, is very important for the purposes of this discussion, and it’s something we’ll come back to.
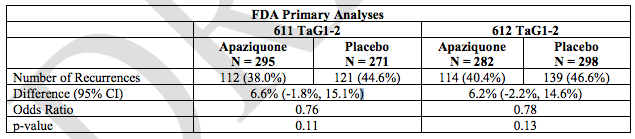
So, Spectrum conducted two pivotal trials designed to underpin its registration application, both of which were pretty much exactly the same. Both investigated the efficacy of the drug in patients with TaG1-2 grade tumors, wit ha single administration immediately post resection, comparing an active arm and a placebo arm on a circa 1:1 ratio. The table below shows the results of these two trials.
The primary endpoint of both trials was disease recurrence, defined as any histologically-confirmed bladder cancer, within the 2-year study period. Both trials failed to demonstrate a reduction in disease recurrence with apaziquone at 2 years. The 2-year rate of recurrence was lower for apaziquone-treated than for placebo-treated patients in both trials. Again, however, there are a few important points to note here.
The first is that the difference between the two arms, in both trials, is just 6.6% and 6,2% respectively. This is far lower than the generally accepted 12% benefit established by way of meta analysis, and is at the very low end of the above mentioned 5-15% spectrum of benefit. The second key point is that the p-values for both trials were way above the generally recognized stat sig value. For an outcome to be considered stat sig, or to put this another way, for us to be able to say that the result seen came about on the back of the input (in this instance, the apaziquone treatment) a p-value of less than 0.05 is required. Anything above this translates to statistical insignificance – essentially, the inability to say that the treatment caused the result. If a trial is non stat sig, any endpoints past the primary are rendered useless.
So how can Spectrum be hoping for an approval based on two failed trials?
Well, the company has pooled the data from the trials and somehow managed to eek out some level of statistical significance, and demonstrated a 7% reduction in recurrence. It’s this 7% that Spectrum is basing its application on. There’s an additional kicker, but it’s a long shot: the company has stated that the drug can be used in patients that have a perforation in the bladder – something that the current off label drugs aren’t suitable for. It suggests in the application that a large number of physicians don’t administer the current off label chemo agents because of this perforation related potential setback, and that an approval for apaziquone would offer a solution for these patients and – in turn – increase intravesical chemotherapy usage. However, in a single, third party, study that recorded the reason immediate intravesical chemotherapy was not given, a deep resection (and therefore possible perforation) was reported in just 9% of patients. That Spectrum is using this 9% as a potential reason for approval is a real straw clutch in our opinion.
These numbers aside, there’s an even more important (and damning) point.
The vast majority of physicians don’t use this sort of treatment (by way of the above discussed off label drugs) because they don’t deem 12% to be a worthy clinical benefit. That’s a 12% reduction potential. Even in the pooled analysis, tacked together by way of an ad hoc, post analysis hypothesis, Spectrum has only managed to demonstrate a 7% reduction. As such, even if the FDA were to approve the drug in this indication, there’s a very low chance of physicians actually using it. To put this another way, if they don’t use a drug that creates a 12% reduction benefit, what would make them use a drug that creates a 7% reduction benefit?
So this brings us to the advisory panel review. As mentioned, the panel voted against approval, on the back of the above mentioned points. Here’s what the agency said to the panel heading in to review day:
The Agency’s primary concern is whether the Applicant has demonstrated substantial evidence of efficacy. Substantial evidence ensures, through well controlled and well-conducted clinical trial(s), that a treatment effect has been identified and is not due to variability in the underlying disease, bias, or chance alone.
- VOTE: Has substantial evidence of a treatment effect for apaziquone over placebo been
demonstrated?
- DISCUSSION: For those who voted “yes” to question 1 that an effect has been demonstrated, please discuss the clinical meaning of the results of studies 611 and 612 (these are the numbers used to reference the trials we’ve already looked at.
All 14 members of the panel voted against the approval of the drug, which renders the second element of the FDA’s questionnaire, the DISCUSSION element, moot. Essentially, the panel said that there was no evidence that underpins an efficacy hypothesis, and that to replace the current off label treatments with a drug that is – at best – two thirds as effective, doesn’t make any sense.
Spectrum is conducting a Special Protocol Assessment right now, aiming to demonstrate the impact of two doses, but the chances of this affecting the outcome are very slim, if not nonexistent.
We probably don’t need to stress our opinion on this one, but for the sake of drawing a conclusion, here’s our take:
Apaziquone is not going to get approved. It’s ineffective and unnecessary, and the kicker of perforation related usage (which wasn’t even brought up as part of the panel questions) is nowhere near a strong enough point to influence the FDA’s decision.
Readers can check out the documentation at the following links: