Q: Please tell us about yourself and your background.
I was appointed Pluristem’s President and Chief Operating Officer in 2014. Prior to that, I served as Pluristem’s Chief Financial Officer and Secretary since 2006, and Executive Vice President since 2013.
Before joining Pluristem, I was the Chief Financial Officer of Elbit Vision Systems Ltd., a public company. Prior to that I served as manager of audit groups of the technology sector at Ernst & Young Israel. I am the Co-Chairman of Israel Advanced Technology Industries (IATI), the largest umbrella organization representing Israel’s high tech and life science industries. I represent Israel’s life sciences industry and have served on the Board of Directors of IATI for the past three years. I also founded and served as Chairman of the “The Life Science Forum”. I hold a bachelor’s degree with honors in business administration and accounting and is a Certified Public Accountant in Israel.
Q: Can you please tell us about your company and the specific problem or challenge that you are addressing with your pipeline?
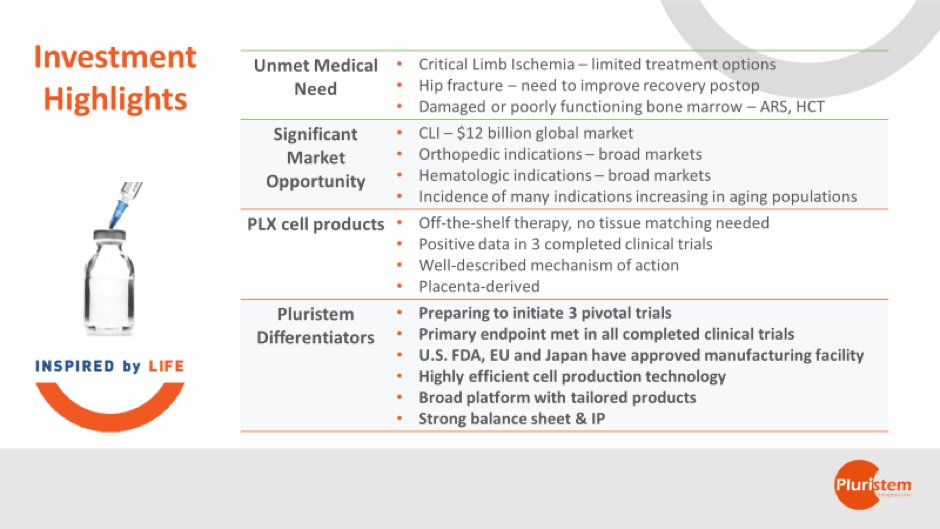
Pluristem is a late-stage biotech company that is developing cell therapies to treat a range of severe diseases with limited treatment options. A number of the diseases we are targeting, such as critical limb ischemia and recovery after surgery for hip fracture, are associated with aging. As our populations age in the developed world, these conditions are impacting an ever increasing number of patients, and are straining healthcare systems and resources. Innovative new approaches are needed to treat the many complex diseases that increase with aging, as current surgical and pharmacological approaches will not be sustainable in the coming decades. Cell therapy, along with other emerging fields, can play an important role in treating chronic diseases in a way that is effective and sustainable.
Q: Can you please tell us a little about the market and the market opportunity?
We have two distinct products in clinical trials that target large markets.
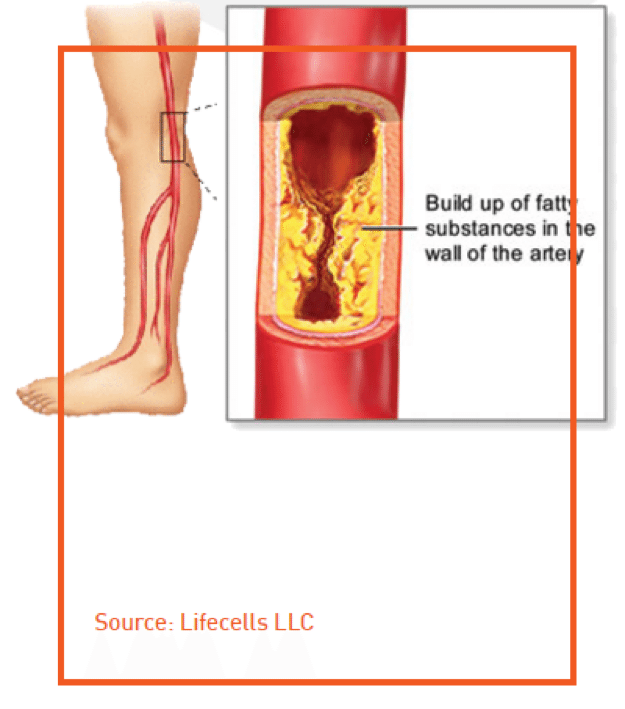
PLX-PAD is our first cell product, and the lead indication with this product is critical limb ischemia (CLI). CLI is estimated to be a $12 billion global market (major markets), with approximately 1.8 million patients in the U.S. and close to 3 million in Europe. We are preparing to start a phase 3 trial in the U.S. and Europe early next year, and data from that one trial could be used to apply for marketing authorization in both key regions.
We are also designing a phase 3 pivotal trial, to be conducted in the U.S. and the EU, to study if PLX-PAD can improve rehabilitation after surgery for hip fracture. PLX-PAD has been shown to trigger the body to effectively regenerate muscle. We completed a very positive phase 2 trial in recovery of muscle strength after hip replacement surgery. It can take from 3-4 months to a year to return to normal activities after surgical repair of a hip fracture, and a large number of patients do not return to fully independent living after rehabilitation. There is also a significant increase in mortality after hip surgery, related in many cases to complications arising from limited mobility.
The lead indication for our second product, PLX-R18, is acute radiation syndrome (ARS). The U.S. National Institutes of Health is currently supporting and conducting a dose selection trial with our cells in this indication, which could serve as the basis for a pivotal trial via the FDA’s Animal Rule pathway. The market here is also potentially very large. We believe that governments around the world would be interested in stockpiling a treatment for bone marrow decimated by exposure to high levels of radiation after a nuclear catastrophe. PLX-R18 is also being developed to treat incomplete engraftment of a hematopoietic cell transplant. A phase 1 trial has been cleared by the FDA and is planned to begin in the coming months.
PLX-PAD secretes a range of therapeutic proteins which pushes the body to grow new vessels to bypass those that are damaged, reduces inflammation in healing tissue, and triggers regeneration of damaged muscle. PLX-R18 cells secrete proteins that target bone marrow regeneration.
Q: Please tell us a little about the technology that drives your pipeline.
Q: What geographic markets are you focusing on currently?
We are active with many regulators around the world including U.S, Europe, Japan, Israel, Australia and South Korea. We have also recently signed a term sheet for a $30M investment with a strategic Chinese partner.
For the large markets like CLI and hip fracture we plan to partner with a larger pharma company to commercialize the product. For ARS we will consider marketing it ourselves since customers covering large markets would most likely be government bodies and we have the bandwidth to handle this type of commercialization.
Q: What are the key benefits and features of your pipeline/drug for the target customer?
Our PLX cell products each target different indications with customized cytokine secretion profiles. Since they are derived from the placenta, the cells have low immunogenicity and immune-modulatory properties, so they can be administered to patients without the need for HLA-matching. This means our “off-the-shelf” products do not need genetic or tissue matching prior to administration, which makes treatment much more convenient in many medical settings.
Q: Who are your competitors?
Cell therapy is an emerging space and so we believe that a rising tide will lift all boats, meaning that we don’t see other companies as competitors in the classic sense. Their success is ours as well because it benefits the space as a whole. Two companies that are working in the allogeneic cell therapy space alongside us are Athersys, Inc. (NASDAQ:ATHX) and Mesoblast limited (ADR) (NASDAQ:MESO). Some key differentiators for Pluristem are that we source our cells from the placenta, and control our own 3D manufacturing that is certified through commercialization by the FDA and EU regulatory agencies as well. In other words we are not dependent on outside partnerships for our manufacturing capacity. Our technology platform also allows us to make many distinct products from these cells.
Right now we have two products expected to enter into three pivotal trials next year: PLX-PAD in CLI, and in mobilization after surgery for hip fracture, and PLX-R18 in ARS, which we expect could be conducted and supported by the U.S. National Institutes of Health pending results of the ongoing dose selection trial that they are conducting.
Q: What advantage does your product offer in contrast to your competitors?
We have several features that set us apart. Some key differentiators are that we source our cells from the placenta, have our own 3D manufacturing and a facility that is certified through commercialization by the FDA and EU. We have also met the primary endpoints for all of our completed clinical trials.
The cells can be used “off-the-shelf” in an office setting, meaning that no manipulation or matching is required before administration, and can be given with intramuscular injections using a standard syringe.
The placenta is a unique raw material source for cells because placental cells are uniquely immunoprivileged. They are missing certain proteins on their cell membranes, so they are not recognized by immune systems as foreign and are not attacked. Mesoblast and Athersys source adult cells from their source in bone marrow cells; their class of cells can also be immunoprivileged, but placental cells are designed, by virtue of their function as a gateway between the maternal and fetal immune systems, to be intrinsically immunoprivileged so that they are not attacked by either the mother’s or the baby’s system. We use cells from multiple placental donors in each of our trials, showing that our cells do not need to be matched and can be a commercial grade product. We are not dependent on one ‘ideal’ donor. Placental cells are robust, multiply rapidly and maintain potency, because they come from the youngest source of adult cells, meaning the placenta at live birth. They have had minimal exposures to viruses or environmental toxins.
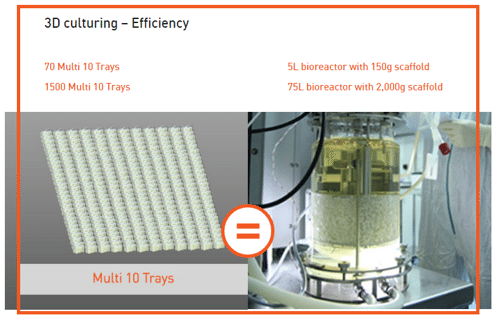
Commercial manufacturing is a critical bottleneck in the space. Many people are now focused on this challenge, because if new technology is needed to produce commercial quantities of cells, one runs the risk of changing the cell characteristics by growing them with different technology. In contrast with others in our space, we have our own FDA and EU-approved manufacturing facility through commercialization meaning if and when our products reach approval, we are already cleared to use our own manufacturing facilities to commercialize them. We have the capacity to make 150,000 doses of commercial grade PLX cells at this time, and to increase capacity we need only scale out with additional bioreactors. We do not need to modify the patented production technology. We can make 20,000 doses from a single placenta, and this efficiency is because of our patented manufacturing technology and our placental cell source.
Q: Tell us about your team.
Our CEO Zami Aberman helped give the initial direction for Pluristem towards cellular therapies and placental cells in particular. Company management team has taken PSTI from a handful of people to a 170 person-company with multiple PhDs and MDs, a high-capacity manufacturing facility that is FDA and EU-certified, and is entering multiple late-stage trials. The company also has a healthy balance sheet thanks to the efficiency of our executive staff and their direction.
Q: How will you succeed in this market?
The cell therapy market is far from saturated. The field is still emerging and there are many complex and multi-factorial diseases such as critical limb ischemia that have limited treatment options and are perfect targets for cell therapy. In addition, many of the indications targeted by cell therapies are most common in older population, and the aging population is increasing significantly in the Western world.
Q: Please shed some light on the various stages of the pipeline and what we can expect from them.
Some upcoming milestones we are targeting:
We are initiating a pivotal Phase III clinical trial of PLX-PAD in Critical Limb Ischemia (CLI) to be conducted in the U.S & EU. In Europe the trial is via the Adaptive Pathways project. We are also initiating a pivotal trial of PLX-PAD cells in CLI in Japan, via the rapid regulatory pathway for Regenerative Medicines, together with a partner.
For the recovery after surgery for hip fracture indication, we are planning to submit and obtain approval of the protocol for a pivotal Phase III trial as well for PLX-PAD. That trial is planned for the U.S. and the EU.
We are looking forward to a data read out from an ongoing dose selection trial in Acute Radiation Syndrome (ARS), being supported and conducted by the U.S. NIH.
A Phase II trial of PLX-PAD for Intermittent Claudication (IC) is ongoing in the U.S, Germany, Israel and South Korea, and enrollment should soon be completed.
We are also looking forward to initiating a Phase I trial in incomplete engraftment of hematopoietic cell transplant for our PLX-R18 product. The FDA has cleared our Investigational New Drug application.
Q: Can you tell us about your cash on hand, burn rate, and upcoming financing rounds?
As of September 30, 2016, we had approximately $29.3 million in cash and cash equivalents, bank deposits, restricted deposits and marketable securities. Our net cash used for operating activities was $3.9 million during our first quarter this fiscal year. Our net burn rate is about $22 million. This year we signed a binding term sheet for an investment of approximately $30 million by a China-based, healthcare-focused investment fund. That puts our cash at around $63 million, or a very high half of the size of the company. We anticipate being well capitalized to conduct the clinical trials planned for initiation in the coming quarters, as well as ongoing R&D efforts to support development of our future products.
Q: Can you tell us about the investment opportunity?
We aim to develop and commercialize effective cell therapies to treat a range of diseases with poor treatment options. We have reported robust clinical trial data in multiple indications for our patented PLX (PLacental eXpanded) cells and are entering late-stage trials in several indications. PLX cell products are grown using our proprietary three-dimensional expansion technology, which enables us to be able to produce commercial-grade cells with batch-to-batch consistency. They are off-the-shelf, requiring no tissue matching prior to administration.
Pluristem has a strong intellectual property position. We own and operate our GMP-certified manufacturing and research facilities, and enjoy strategic relationships with major research institutions.