
VBL Therapeutics AKA Vascular Biogenics Ltd (NASDAQ:VBLT) has a revolutionary approach to curing cancer. Their rich pipeline combines proven scientific approaches including gene-therapy techniques with an immunological angle, in order to target the vasculature feeding the tumor and induce an anti-tumoral immune response.
We spoke to Dror Harats, founder and CEO of VBL Therapeutics. Here is what he had to say.
Q: Please tell us about yourself and your background?

I received my M.D. from the Hadassah Medical School, the Hebrew University of Jerusalem, Israel. Following a residency in Medicine at the Hadassah Medical Center in Jerusalem, I have completed my fellowship in pulmonary medicine and research in Molecular Genetics at The University of California in San Francisco (UCSF). I was then a Visiting Scientist at Syntex Discovery for three years.
In addition, for the last 15 years I chair the IRB committee of the Sheba medical center, supervising about 2000 clinical trials annually.
Q: Tell us about your company and the specific problem or challenge that you are addressing with your pipeline?
VBL Therapeutics is an innovative biopharma company focused on development of first-in- class therapies for cancer. Treatment of cancer is challenging – tumors carry different mutation, and activate various pathways to evade the immune system and recruit new vasculature to supply their needs. Knowing that it is almost impossible to eradicate every tumor cell in the body, we developed unique approach to block the ability of tumors to grow, aiming at the extension of patient’s survival. Our approach combines gene-therapy techniques with an immunological angle, in order to target the vasculature feeding the tumor and induce an anti-tumoral immune response.
Q: Tell us a little about the market and the market opportunity?
We developed an innovative viral-immuno-oncology therapy which is targeting the blood vessels of solid tumors and leads to tumor starvation. This therapeutic approach is implemented through our VB-111 drug candidate that has demonstrated efficacy in three phase 2 trials, in recurrent GBM, ovarian cancer and thyroid cancer. These represent only the
tip of the iceberg for a potentially broad range of solid tumors, applicable to millions of patients worldwide. If approved, VB-111 has the potential of becoming a blockbuster drug.
Q: Please tell us a little about your technology that drives your pipeline?
The heart of our technology is a proprietary tissue- and condition-specific regulatory element (“promoter”) that is activated only in angiogenic, proliferating endothelial cells of blood vessels. It is so specific, that we can attach to it a very toxic gene and deliver it systemically, and it will destroy tumor blood vessels without harming normal vasculature or other organs. The same promoter-engine can be used in various ways, to destroy blood vessels for oncological applications, or to build new stable blood vessels in cases of ischemia. In order to deliver this therapeutic element into the cells, we use a non-replicating, non-integrating flu- like engineered virus, that help triggering the immune system in the tumor environment. Therefore, our drug candidate VB-111, , acts both to destroy tumor blood vessels and turn on anti-tumoral immune response, which was evidenced histologically via infiltration of CD8 T-cells and apoptosis of tumor cells. VB-111 is currently in a Phase 3 trial that will be sufficient, if successful, to achieve regulatory approval (SPA) and that is also designated for fast-track by the FDA
Take a look at this video for an in depth description of how VB-111 works:
https://youtube.com/watch?v=kOoFPTM-5QA%3Frel%3D0%26autoplay%3D1
Q: What geographic markets are you focusing on currently?
Our current clinical activity is in the US, Canada and Israel, but we intend to develop VB-111 globally, in collaboration with strategic partners.
Q: What markets will you focus on in the near future and what is your plan to penetrate these markets?
Our major focus is to pursue regulatory approval for VB-111 in the US, through rGBM which is a devastating disease with a huge unmet need. Later on, we intend to expand to additional indications, such as ovarian cancer , for which we have already obtained promising Phase 2 data and other solid tumors, for which we have strong signal from the preclinical development and the Phase 1. Following US approval, we intend to collaborate with strategic partners and/or local distributers to commercialize VB-111 globally.
Q: What are the key benefits and features of your pipeline/drug for the target customer?
First, and most importantly, we have seen profound effects on patient survival both in rGBM and platinum-resistant ovarian cancer, even in patients whose tumors failed to respond to prior therapies. VB-111 has an excellent safety and tolerability profile, and therefore it can also be combined with other treatment modalities. In addition to the efficacy and safety profile, VB-111’s is patient friendly. Its administration and frequency of dosing, once every 2 months via a simple IV infusion, is very convenient to the patient and the physician.,.
Q: Who are your competitors?
In the GBM field, most therapies are focused on newly-diagnosed patients. Patients who had a recurrence (following surgery, radiotherapy and chemotherapy) have very limited options. The standard of care in the US is Avastin. It is still early to say whether immunotherapies will be efficient in rGBM, but in general, many therapies fail to show efficacy in brain tumors due to low penetration through the blood-brain-barrier.
Q: What advantage does your product offer in contrast to your competitors?
Avastin® (Roche/Genentech) is a well-known anti-angiogenic drug with a multi-billion market size. However, Avastin blocks one anti-angiogenic factor (VEGF), while the tumor cells have redundancy and can overcome VEGF blockade by secreting one or more alternative pro-angiogenic factors. The MOA of VB-111 is completely differentiated from Avastin or TKIs, and is not limited to a certain factor or mutated pathway. In fact, in ovarian cancer, we have seen responses with VB-111 even post failure on Avastin and TKIs, including in women whose tumors were platinum-resistant/refractory. The added value coming from VB-111’s immune effect further enhances its anti-tumoral activity. Currently, there are very limited options for recurrent-GBM or platinum-resistant ovarian cancer patients, especially treatments that can prolong patients’ survival.
Q: What makes your pipeline stand out?
We see VB-111 as the first candidate of our Vascular Targeting System (VTSTM) platform, which can be utilized for various applications. VB-111 itself has a pipeline-in-a-product potential, as a shelf product applicable for multiple solid tumor indications.
Q: Tell us about your team?
We have seasoned and well experienced professionals who have worked at large pharma companies in the US and abroad. Our board of Directors, which is led by Bennett Shapiro, MD who headed Merck Research Laboratories for many years, comprises highly experienced and competent people like Drs. Ron Cohen (Acorda) and Ruth Arnon (Weizmann institute; inventor of Copaxone). We also work together with excellent advisors, and our Scientific Advisory Board is led by Rachel Humphrey, a key leader in the immuno-oncology field.
Q: How will you succeed in such a saturated market?
We currently target orphan indications, coming as a late line of therapy following failure of previous therapies. Our ability to show OS benefit in such setting shall help the approval of VB-111 for those indications. Later on we may expand our treatment to newly-diagnosed patients and to larger indications.
Q: Please shed some light on the various stages of the pipeline and what we can expect from them?
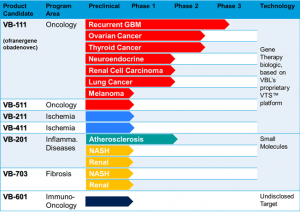
The VTS, or the proprietary tissue- and condition-specific regulatory element, that I have described to you earlier, and its lead candidate, the VB-111 us the latest stage
asset of VBL. As mentioned VB-111 is in pivotal trial, and if succeeds will be ready
for BLA during 2018;
VB-601, a tumor specific membrane protein, will be disclosed to the public early
2017, is an early stage immune-oncology therapy, in preclinical development; and:
The Lecinoxoids: a family of phosphor-lipid small molecules. These molecules inhibit the TLR2 and TLR4 pathway of the innate-immunity system, as well as attenuate monocytes migration. This, Phase 2 ready technology, demonstrated activity in atherosclerosis inflammation and Nash.
Q: What is your focus for the next 6-12 months?
- We expect to report full data from our Phase 2 trial of VB-111 in thyroid cancer, including Overall survival data by the end of 2016
- Plan to promote a potential-registration trial for VB-111 in ovarian cancer during 2017
- Expect interim data for the Phase 3 pivotal study in rGBM in 1H 2017
- Expect full data from the Phase 3 pivotal study at YE17/beginning of 2018.Q: Can you tell us about your cash on hand, burn rate, upcoming rounds?On June 30, 2016 we had $51.6M in cash, which takes us into 2019. This covers completion of the Phase 3 pivotal study in rGBM and the potential-registration trial for VB-111 in ovarian cancer.Q: Can you tell us about the investment opportunity?VBL is not actively seeking investments at this point, as we are well funded into 2019. That being said, we have many plans that we would like to implement, and we value smart-money and qualitative long-term investors.Q: Anything that you would like to add?
Thank you for the opportunity to describe VBL Therapeutics. For additional information please see www.vblrx.com . Please feel free to contact us via our IR firm – Lifesci Advisors.




