This report covers:
- Kempharm KP201/APAP advisory review.
- Novo Nordisk’s upcoming FDA review of Xultophy.
- Sanofi’s upcoming FDA reviews of Lyxumia & LixiLan.
KemPharm Inc (NASDAQ:KMPH) KP201/APAP Advisory Panel Review, May 5 2016.
In our coverage of the week to come, published earlier this week, the headline data was the pending advisory panel review of KemPharm Inc (NASDAQ:KMPH) KP201/APAP. The drug, which for the purposes of the new drug application (NDA) took the commercial name Apadaz, is an abuse deterrent opioid treatment with a target indication of acute pain.
For information on the science behind the drug, and KemPharm’s financial standing, take a look at last week’s report. To briefly introduce Apadaz, however, it’s essentially a reformulation of two currently available opioid drugs – benzhydrocodone hydrochloride and acetaminophen – that has what’s called a ligand attached to it. The ligand serves to hold the drug in an inactive state until it hits the gastrointestinal tract, and in doing so (or at least, KemPharm had hoped), makes the drug abuse deterrent. Why? First and foremost, because it cannot become subject to the traditional methods of use – crushing, dissolving etc. Secondarily, it’s inactivation makes it very difficult to overdose on.
So an FDA panel got together on May 5, 2016, to discuss the relative merits of Apadaz as supported by its phase 3 NDA data. Unfortunately for KemPharm and its investors, things didn’t work out quite as well as initially planned.
The advisory panel voted in favor of approval, with a 16 to 4 favorable position. However, when it came to the abuse deterrent labeling, the panel voted 18 to 2 against abuse deterrence being listed as a quality of the drug.
So if the drug is approved, which is looking highly likely based on the panel review, why is this not a good thing for KemPharm? The way to think of this is the abuse deterrent element of the drug is its efficacy. That benzhydrocodone hydrochloride and acetaminophen work to reduce acute pain was never in question, hence their current standard of care (SOC) status in just this indication. What was under scrutiny in this trial, and subsequently, in the review meeting, was the drug’s abuse deterrent qualities. Essentially, is it an improvement on the current SOC, with improvement defined as some sort of differentiation – specifically, that users cannot overdose or abuse the active compounds that make up the drug. This differentiation is the efficacy in this situation, and with the review panel voting against a label that highlights the drug as abuse deterrent, they are essentially saying that the drug is not effective.
For KemPharm, of course, this is disastrous. The company has spent many millions of dollars developing a drug that now looks as though it is only going to get approval as an acute pain opioid, with no discernible benefit over the currently available standard care treatment.
There are a couple of brand name players in the space that command large revenues, and also a flurry of readily available generic formulations. As such, without an abuse deterrent label, Apadaz is essentially worthless to KemPharm.
Market Reaction and Trading Strategy
Not surprisingly, markets sold off on the company in response to the advisory panel’s decision release. As things stand, KemPharm’s market capitalization is down close to 55% on its pre-announcements valuation. This selloff reflects the fact that without abuse deterrence properties, the company’s pipeline is effectively composed of generic pain therapies, and in turn, is not likely to generate much in the way of sales going forward.
Of course, the panel review is not the final say on the situation, and this presents two very different trading strategies as available options. The aggressive risk-taking trader might look at the situation as an opportunity to get into KemPharm ahead of PDUFA at a 55% discount to last week’s pricing, on the assumption that the agency will overlook the review panel’s decision and go ahead with an abuse deterrent labeling approval. That there is an inherently large risk in this approach need not be pointed out.
The second strategy is a short KemPharm in anticipation of an agency acceptance of the review panel’s decision, and a resulting decline on the labeling element of the treatment.
What’s important to recognize here is that whether or not Apadaz gains approval is not important anymore. The only thing that matters now, is whether the drug gets an abuse deterrent labeling. Even with the approval, without the label, the drug is essentially valueless. As a result, id this situation plays out as the panel review suggests it might, KemPharm will lose additional market capitalization come June 9 (PDUFA).
Two weeks ahead: May 24th – May 26th 2016
Nothing too exciting is happening next week in terms of FDA approvals or panel reviews, but two weeks from now will be very active. So this week we will skip forward another week and cover a big diabetes battle between Novo Nordisk A/S (NYSE:NVO) and Sanofi SA (NYSE:SNY). AstraZeneca PLC (NASDAQ:AZN) and Sarepta Therapeutics (NASDAQ:SRPT) will again be featured in two weeks, but we’ll cover that next week.
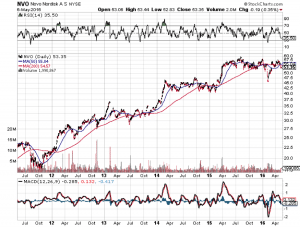
Novo Nordisk A/S (ADR) (NYSE:NVO)

On May 24, an FDA panel will review a drug combination branded as Xultophy, which is a combination of insulin degludec and liraglutide. Both insulin degludec and liraglutide are approved in the US, but the combination of the two drugs into one does is what’s under review. Doctors can still prescribe both separately and often do, so this is only a matter of efficiency and cost saving rather than the blasting open of new markets. Novo Nordisk’s insulin degludec is marketed as Tresiba, and liraglutide as Victoza. Together they treat both diabetes and obesity, which often come together.
Science
Novo Nordisk’s bread and butter is Victoza. Victoza, or liraglutide, is a GLP-1 receptor agonist, for glucagon-like peptide-1. Normally, beta cells in the pancreas react to GLP-1 agonists by producing insulin. We normally produce GLP-1 agonists naturally. In diabetics, either the native GLP-1’s don’t work, the pancreatic beta cells don’t respond to it, or the pancreatic beta cells are too far gone to react to it. It is not insulin itself, but a signal for the pancreas to secrete it.
If the human body can produce its own GLP-1 agonists, what’s the purpose of Victoza? The difference is that Victoza is not as easily metabolized and stands up to the body’s protein digestion, so it can get to its target in the pancreas which then produces insulin. Diabetics attack their own endogenous GLP-1 so it can’t get to the pancreas in time to induce the pancreatic beta cells to produce insulin. Victoza does. The drug is also known to help regenerate the beta cells of the pancreas themselves so they can more easily produce insulin. What makes the drug so popular is that it also inhibits gastric emptying, meaning the stomach stays fuller and feels fuller for longer, which induces people on Victoza to eat less.
There is one more major advantage to liraglutide, and that is, it itself is activated by glucose, meaning it is glucose-dependent. If the level of glucose in the blood is high, liraglutide stimulates beta cells to produce insulin. If it is low, the liraglutide remains inactive. This leads to controlled and self-regulated blood sugar levels, with little risk of hypoglycemia (low blood sugar) if a patient takes too much or doesn’t eat enough sugar with the dose.
Together, liraglutide leads to a combination of weight loss and self-regulating blood glucose levels, which are ideal for diabetics.
As for Tresiba, or insulin degludec, this is the more classic insulin analogue that replaces the insulin that is meant to be produced by the pancreas. Some diabetics have working beta cells that simply cannot be stimulated enough to produce the required levels of insulin. For them, liraglutide is enough. Others have damaged bet cells that wouldn’t produce enough insulin even if stimulated by the GLP-1 agonists. They need additional insulin support. Hence the combination of Tresiba and Victoza into Xultophy, which will be reviewed by the FDA on May 25th.
Insulin degludec is of course an insulin but it has an extra molecule attached to one of its amino acids called hexadecanedioic acid. Hexadeca meaning 16 carbons with carboxylic acid groups at either end. These molecules form chains between the insulin proteins so they link up under the skin after injection. The acid molecules are slowly broken down so that the insulin is gradually released from the chain. The gradual release helps patients keep from getting hypoglycemia from an insulin shock that quickly reduces blood sugar levels and can lead to fainting and general fatigue. The mechanism of release is different from insulin glargine, or Sanofi SA’s (NYSE:SNY) Lantus. Lantus is crystallized insulin that slowly become active in the blood when the crystals dissolve into molecular insulin.
Data
The combination trial of insulin degludec and liraglutide trialed 557 patients, one arm taking the combo plus metformin and the other taking insulin glargine plus metformin. Metformin is a common diabetes treatment that stops the liver from producing extra glucose. Over the first 16 weeks of treatment, the degludec/liraglutide arm saw a drop in average 3 month blood glucose levels (HbA1c) from 8.4% to 6.6%. The glargine arm saw a drop from 8.2% to 7.1%. By 26 weeks, the degludec arm had demonstrated statistical superiority to glargine.
Further, the degludec arm saw average weight loss of 1.4 kg, and the glargine group gained 1.8 kg on average.
While more adverse events were reported in the degludec/liraglutide arm than the glargine arm (79 vs 18) the side effects weren’t serious, mainly upset stomachs.
The main disadvantage of the trial was that it was not long term. There is a small chance the FDA will want a longer trial in its review, but given that both drugs are already approved separately and the combination is already approved in Europe, don’t expect the FDA to put up much of a fight here.
Market
Type II diabetes affects 29 million people in the US and 415 million people worldwide. 600 million suffer from obesity on top of that. Novo Nordisk already has a 28% market share in the global diabetes market, selling DKK 85.6 billion last year for its diabetes segment, or $13.12 billion. Peak sales for Xultophy are estimated at about $1 billion a year, but the question is whether sales will cannibalize from Victoza sales.
Novo Nordisk doesn’t think so because Victoza only has about a 5% market share of the total diabetes market. That’s a lot, but it’s not overwhelming. The company believes Xultophy will attract the more acute diabetes cases for which liraglutide is not enough and they need extra insulin support. Buying both drugs separately is expensive and annoying, requiring two injections every day. Combining them will bring some sales from those who are currently taking glargine, so hopefully for Novo Nordisk there won’t be too much overlap.
Reaction
Don’t expect too much reaction from a positive FDA panel review of Xultophy. It is pretty much expected, and there is little reason for the FDA to reject the drug. It could at worst ask for more confirmatory studies on long term safety which have yet to be done, but this probably won’t happen. Both drugs are already approved, the combination is approved in Europe, and the data are pretty good.
A complete response letter for whatever reason could hit Novo Nordisk shares somewhat, but not long term, maybe a 3 to 5% at most. A positive review could give shares a small bump higher, but after that the market will continue doing what it was doing before.
Valuation and Trading Strategy
Novo Nordisk has a very strong diabetes portfolio, perhaps the strongest in the world. Its earliest upcoming patent expiration is 2022, excluding Victoza’s Chinese expiration in 2017. China accounts for 9.6% of all sales. The company has a high P/E of 25.5 but its incredible growth makes that number deserving. Novo Nordisk is on its way up as the global diabetes leader, while Sanofi looks to be on its way down struggling with shrinking Lantus sales and biosimilar competition due to patent expiry.
The biggest risk for Novo Nordisk has nothing to do with its products, but the structural stability of the health care industry. Its fastest growing market is the US, and one warning sign of potential problems came from Germany last year. In 2015, Novo Nordisk had to discontinue marketing Tresiba in Germany because pricing negotiations fell apart there. That there need be a group of bureaucrats deciding what a product should be priced at rather than free market supply and demand is a consequence of the government controlled and centralized healthcare industry. Not that price negotiations will fall apart in the US over this, but the systemic risks of being so concentrated in a single disease that is so heavily reliant on government programs like Medicare mean that any risk to Medicare can put severe pressure in earnings.
This hasn’t happened yet, but Medicare is not a sustainable program long term. Those who hold companies where the vast majority if their revenues come from programs that are unsustainable need to keep a close eye on the programs that sustain their companies. This includes Novo Nordisk.
Those comfortable with these long term risks can take a position now and if the FDA delays approval for Xultophy, add to it on the dip.
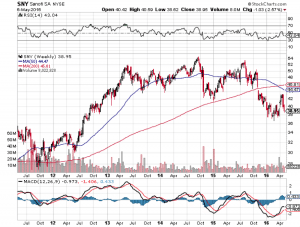
Sanofi SA (ADR) (NYSE:SNY)
Also On May 25th, Sanofi’s Lyxumia (lixisenatide) will be under FDA panel review, as well as LixiLan, a combination of lixisenatide and Lantus (insulin glargine). These are parallel to Novo Nordisk’s Triseba and Victoza. Triseba is parallel to Lantus, and Victoza to Lyxumia.
Science
Lixisenatide is Sanofi’s version of a GLP-1 receptor agonist. The science is the same as liraglutide. The molecule is slightly different but has a similar effect on the beta cells of the pancreas and gastric emptying. Insulin glargine, though, is different from insulin degludec in the way that the extended release is achieved. Degludec achieves it by linking the molecules together by a long acid chain, discussed above. Glargine achieves it by being delivered in crystallized form. The crystals slowly dissolve and become active insulin.
Data
The data on Lixisenatide are not as impressive as with liraglutide, but still good. Sanofi had intended to file for FDA approval much earlier but suspended its efforts in 2013 when internal data showed a cardiovascular risk in high risk patients. Phase III study results on that high risk group were released in June 2015 and showed noninferiority to placebo, but not statistical superiority. That basically means that lixisenatide was proven to make matters worse in high risk cardiovascular patients, but it also did not improve cardiovascular risk either. That’s OK. As long as the drug is shown at least not to exacerbate cardiovascular issues, it will probably be approved.
The earlier data is from 2011 and showed average weight loss for lixisenatide patients at .89 kg, and a lowering of HbA1c (the three month blood glucose average measure) from 8.6% baseline to 6.96% on patients taking both insulin glargine and lixisenatide. That’s a decrease of 19%, a little less than the 21.5% decrease shown for liraglutide/degludec, and a little less weight loss as well.
It looks like the competition between the two combination drugs, assuming both are approved, will be personal preference. Nausea and vomiting are common side effects of these drugs and some people will react better to certain combinations than others. How that will play out in the market remains to be seen.
Market
Since LixiLan is the same drug as Xultophy, just a different variation of it, the market is the same as above. Sanofi sold €38M of Lyxumia in 2015 so it is not as big of a hit as liraglutide, but time may change that as the European market has more time to absorb both drugs. Sanofi had the same problem as Novo Nordisk with its negotiations with the German health authorities and had to discontinue lixisenatide there.
Reaction
Lantus, Sanofi’s insulin glargine, has seen its sales bleed with biosimilar competition and more Lantus copies will come on the market this June, so approval for Sanofi is more critical than it is for Novo Nordisk. It is generally recognized that Sanofi is in some trouble, having failed in its collaboration with MannKind Corporation (NASDAQ:MNKD) on Afrezza, an inhalable formulation of insulin. Sentiment on Sanofi is already down and a negative response from the FDA panel could bring it down further, especially because it needs some good news for its diabetes pipeline. It is likely that they will get approval for Lyxumia at least, and possibly also for the combination LixiLan.
Approval for LixiLan is especially important because it will help bring back some lost sales from Lantus. Patients can take generic insulin glargine starting in June, but if they want the combination, they’ll have to buy the brand. If LixiLan is approved, Sanofi could jump 5 to 10% on the news, perhaps even more considering Sanofi’s share price is depressed to 2012 levels.
A negative review, though unlikely, will hurt Sanofi, possibly taking it to its lows around $37 a share. A dip like that though would not be sustained for long because Sanofi is not as heavily dependent on diabetes as is Novo Nordisk. Sanofi’s portfolio is more balanced even though Lantus is its biggest seller.
Valuation and Trading Strategy
Sanofi can be considered a buy regardless of the FDA panel’s response to LixiLan and Lyxumia. If they say no, there will be a dip and Sanofi will be around its lows with a biotech sector rebound looking likely. If they say yes we’d see a respectable jump in shares. Novo Nordisk’s combo may beat LixiLan in the market, but patients will have their preferences and LixiLan will not be shut out. The stock is a buy now for a nice jump on approval, but if LixiLan is rejected or delayed, the resulting dip should be bought in any case.
Written by: Rafi Farber, Samuel Rae and other Market Exclusive contributors.