Oncology drugs have been a hot topic over the past six months. A number of triple digit million-dollar deals have come out of the space, and incumbents in the arena have dominated news media. However, there are still a number of small cap opportunities that remain under the radar. One such opportunity is Synta Pharmaceuticals Corp. (NASDAQ:SNTA). With a market capitalization just shy of $300 million at last close, the company is very much a development stage entity. As such, there are inherent risks associated with an allocation. Having said this, it has quietly navigated its way through both a proof of concept phase I and an efficacy rooted phase II, and as we head into the second half of this year, is poised to report phase III readouts on a lung cancer indication. Let’s have a look at the company and try to ascertain whether now is a good time to gain exposure.
Lead Candidate
Synta’s current pipeline includes three primary treatments – Ganetespib, STA-12-8666 and Elesclomol. The first two are what is called an Hsp90 inhibitor (which we will look at the little more detail shortly) and the third is a Mitochondria Metabolism Inhibitor. For the purposes of this report, we will focus purely on the former of the three candidates, as this is the one that will drive Synta’s market potential over the coming 4 to 5 years.
The Science
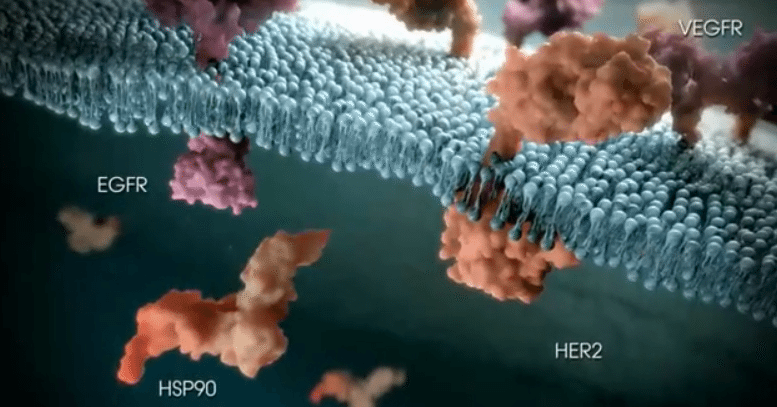
As mentioned, Ganetespib is an Hsp90 inhibitor – meaning it inhibits Hsp90, a type of protein that serves as a “chaperone” protein. Chaperone proteins do many different things, but to keep things simple, think of them as a sort of protein mold. Client proteins feed into chaperone proteins, and chaperone proteins “form” the client proteins into the task performing proteins they are designed to be. There is a nice mechanism of action video here that visualizes this process. Ganetespib attaches to Hsp90 proteins and blocks them from interacting with client proteins. Tumor cells are (reportedly) more heavily reliant on Hsp90 proteins for both proliferation and survival. Therefore, the theory goes that by inhibiting the interaction between client and chaperone, Ganetespib is inhibiting the result of any potential interaction. The result of the interaction, in this instance, being proteins that contribute to tumor cell proliferation. The beauty of this sort of approach is that it is not limited to one form of treatment. We’ve seen markets get excited about BRAF, HER2 and EGFR inhibition treatment already this year. Cancers associated with all these gene mutations are heavily reliant on Hsp90 interaction, so in theory the treatment can supersede these subsections and serve as an overarching fix all. Obviously, this is further down the line, but the potential is there – at least from a scientific perspective.
Clinical Progress
Synta is currently targeting a non-small cell lung cancer indication, and as we have mentioned, is well underway with a phase III. The trial sees Ganetespib tested in combination with a current chemotherapy drug called docetaxel, currently marketed by Sanofi (NYSE:SNY) as Taxotere, versus docetaxel alone. A phase II study designed to determine a phase III population completed last year, with progression free survival (the primary endpoint) for the combination therapy outpacing that of the single therapy by more than 55% – or 5.3 months versus 3.4 months.
From this phase II, physicians selected just under 850 patients for a phase III enrolment, which is currently testing towards a primary endpoint of overall survival; again, using combination versus single therapy. As mentioned earlier on, interim analysis will hit markets during the second half of this year, with final data slated for the first half of next. Both of these events could be real volatility drivers from a market capitalization perspective. If progression free survival (a secondary endpoint that will likely be addressed in the interim analysis) can fall in line with the results we saw at phase II, there could be real upside potential short-term. After that, all eyes will be on overall survival in the final data.
Financial Overview
From a financial perspective, the company has a little over $95 million cash on hand, which it expects to carry operations through to the second half of 2016. Current and historic burn rate averages out at between $2.5 and $3.5 million, but this is not really indicative of an ongoing equivalent as the next couple of years will likely be more expensive from an operational perspective than the last. This said, with no debt and a phase III underway further capital raises should be a problem (so long as we see promising interim analysis).
Takeaway
The takeaway here is that Synta is one to watch. Investors with an appetite for risk may look to take a position ahead of interim analysis, but progression free survival can be notoriously volatile short-term. As a result, what the interim analysis reveals may not be indicative of the final results. For this reason, for more risk averse investors looking to mitigate their exposure somewhat, a position in anticipation of final data mid-2016 could be a rewarding allocation.